Abstract
Approximately 25% of MDS patients (pts) progress to AML; 15% of those with lower-risk, and up to 40-50% of those with higher-risk disease. Predicting those who are likely to progress to AML early in their disease course could directly impact treatment decisions: lower-risk pts with higher risk of AML transformation may be offered transplant, while higher-risk pts may be considered for AML-like therapy.
In this study, we developed a genomic model that evaluates mutational patterns and their association with AML progression. Development of this model mimics Netflix or Amazon's recommender system in which customers who bought products A and B, are likely to buy C: pts who have a mutation in genes A and B, are then likely to progress to AML.
Clinical and genomic data ofMDS pts diagnosed between 1/1996 and 9/2016 were analyzed. A panel of 60 gene mutations obtained by next generation targeted deep sequencing was included. Association rules using Apriori algorithm was used to study the relationship between multiple genes/cytogenetic abnormalities and AML progression in an unbiased approach. Association rules are a machine learning method that uncovers relationships between variables in a given dataset. Rules with highest confidence (>90% that an association exists) and highest lift (strength of the association) were chosen. Univariate and multivariate analyses were used to evaluate the impact of mutations on AML progression, adjusting for covariates (age, IPSS-R).
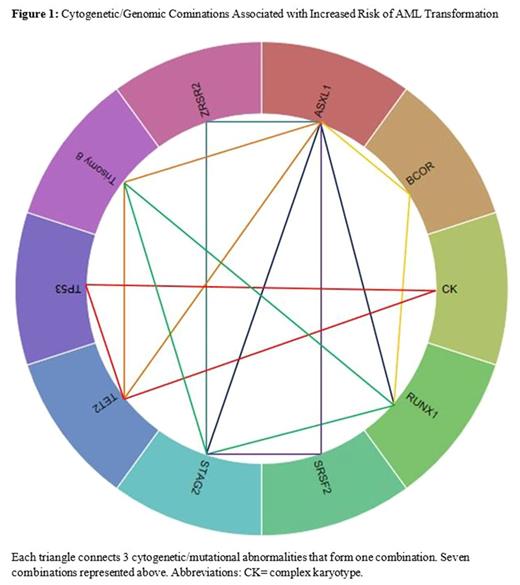
A total of 527 pts with lower-/higher-risk MDS were analyzed, of those 105 (20%) progressed to AML, with a median time to progression (TTP) of 13 months (interquartile range, 6.3-29.8). Median age at diagnosis was 67 years (range, 19-99) and 38% were female. Risk categories by IPSS-R included: 78 (15%) very low, 200 (38%) low, 95 (18%) intermediate, 98 (18.5%) high, and 56 (10.5%) very high; IPSS-R cytogenetic risk groups were 15 (3%) very good, 331 (63%) good, 87 (16%) intermediate, 37 (7%) poor, and 57 (11%) very poor. The most commonly mutated genes were SF3B1 (14%), ASXL1 (13%), TET2 (12%), SRSF2 (11%), DNMT3A (10%), STAG2 (9%), and TP53 (8%). Per IPSS-R, 14% of pts with very low/low, 21% with intermediate and 30% with high/very high categories progressed to AML. Univariate analyses identified the following cytogenetic and mutational abnormalities to be associated with AML progression: complex karyotype (CK, P=.019), ASXL1 (P=.023), DNMT3A (P=.011), FLT3 (P=.031), PHF6 (P=.018), PTPN11 (P=.037) , RUNX1 (P=.001) , STAG2 (P=.023) , TET2 (P=.047) , U2AF1 (P=.038), and ≥3 gene mutations( P=.002), whereas SF3B1 was associated with lower risk of AML progression (P=.025). In multivariate Cox regression analyses, including significant mutations, age (P=.5) and IPSS-R scores (P <.001), only RUNX1 (HR2.8 ; 95% CI 1.6-5.3; P= .001), and ≥3 gene mutations (HR=1.65; 95% CI 1.1-2.5; P= .021) were independent factors that associated with AML progression, while SF3B1 (HR=.417; 95% CI .205-.851, P=.016) was associated with lower risk of progression. Association rules identified the following combinations of cytogenetic/genomic abnormalities for AML progression: (ASXL1, RUNX1, STAG2/BCOR), (ASXL1, STAG2, ZRSR2/SRSF2), (ASXL1, TET2, Trisomy8 ), (RUNX1, STAG2, Trisomy 8 ), ( CK , TP53, TET2) . These combinations were associated with a 5 month reduction in time to AML progression.[Figure 1]
Using genomic data to reliably identify patients at highest risk for progression to AML early in their disease course can dramatically alter treatment recommendations. Independent factors such as RUNX1 and ≥3 gene mutations predicted for AML progression, while SF3B1 was associated with a reduced risk. Certain cytogenetic/genomic abnormalities were associated with shorter time to AML progression. This data suggests that MDS pts with ≥3 genomic abnormalities or certain genomic combinations could be offered more aggressive therapy early in their disease course including those with lower risk disease.
Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees. Gerds: Incyte: Consultancy; CTI BioPharma: Consultancy. Advani: Takeda/ Millenium: Research Funding; Pfizer: Consultancy. Maciejewski: Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Speaker Fees; Apellis Pharmaceuticals: Consultancy; Ra Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal